Abstract
Introduction: Despite advances in cytotoxic and targeted therapies, recurrent disease remains the most significant obstacle to long-term survival in patients with childhood and adult leukemias. As part of our efforts to identify therapies that can be repurposed for immediate use in patients with leukemia, we interrogated a library of all available antibody-drug conjugates (ADCs) whose targets are expressed in leukemias (AML or ALL). A list of targets with available ADCs was merged with transcriptome data from over 3000 pediatric and adult leukemias (AML and ALL) to identify targets that are expressed in a large cohort of leukemias with immediate therapies for repurposing. The transcriptome data set included nearly 2000 pediatric AML cases sequenced as part of Target Pediatric AML (TpAML), 419 adult AML cases from TCGA LAML and the Beat AML program, as well as 853 ALL cases from COG and St. Jude trials. In this repurposing endeavor, CD74 emerged as the most expressed transcript in AML and ALL. CD74 encodes for a cell surface protein that associates with the class II major histocompatibility complex and is involved in the regulation of antigen presentation for immune response and B-cell differentiation. STRO-001 (Sutro Biopharma) is a CD74-directed, site-specific ADC developed for the treatment of multiple myeloma and lymphomas. Given broad expression of CD74 in leukemias, we evaluated the efficacy of STRO-001 in AML and ALL preclinical models.
Methods: To characterize CD74 expression, RNA-seq data obtained from pediatric and adult AML and ALL patients was examined. Cell surface expression of CD74 was determined by flow cytometry using PE labeled anti-human CD74 antibody. The CD74-targeting ADC (STRO-001) was obtained from Sutro Biopharma.The preclinical efficacy of STRO-001 was evaluated against AML and ALL cell lines and patient samples expressing various levels of CD74 in vitro and in vivo. For in vivo studies, AML and ALL cell lines were transduced with GFP/Luciferase construct, and GFP+ cells were injected intravenously into NSG mice. Leukemia burden was measured by bioluminescence (IVIS) imaging weekly.
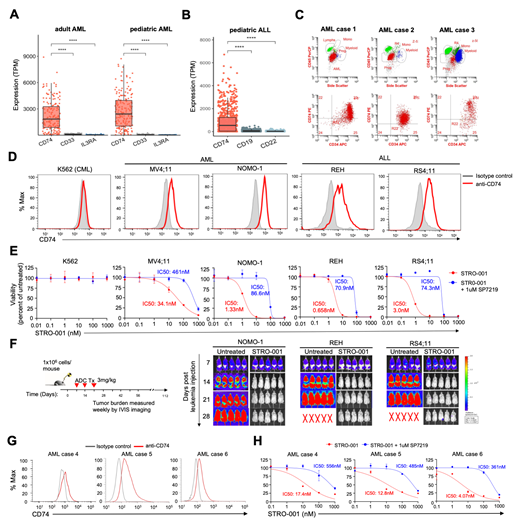
Results: Transcriptomics analysis showed CD74 expression in a majority of adult and pediatric AML (>99% of cases) and at a much higher level compared CD33 and CD123 (targets currently developed for AML, Fig. 1A). CD74 is also broadly expressed in pediatric ALL, with a significant increase in expression observed compared to CD19 and CD22 (known targets in ALL, Fig. 1B). We confirmed that CD74 is expressed on the cell surface of AML blasts in primary patient samples (Fig. 1C) as well as AML and ALL cell lines (Fig. 1D). Given confirmation of cell surface expression of CD74, we investigated whether targeting CD74 can effectively eliminate leukemia cells. We evaluated the in vitro cytotoxicity of STRO-001 against K562 (a CML cell line that does not express CD74), AML cell lines (MV4;11 and NOMO-1), and ALL cell lines (REH1 and RS4;11) with varied CD74 expression. STRO-001 demonstrates target-specific cytotoxicity against CD74-expressing AML and ALL cell lines, but not K562 cells (Fig. 1E). STRO-001 exhibited high potency in CD74 expressing cells, with IC-50s of 41nM (MV4;11), 1.3nM (NOMO-1), 0.7nM (REH-1) and 3nM (RS4;11). In vivo studies in NSG mice transplanted with AML and ALL cell lines showed high potency. Treatment with STRO-001 at 3mg/kg once a week for 3 weeks effectively eradicated the leukemia in NOMO-1, REH-1, and RS4;11-bearing xenograft mice, while disease progression was observed in untreated control mice (Fig. 1F). We further evaluated the efficacy of STRO-001 in primary patient samples. Primary leukemia specimens from 3 patients with varied CD74 expression (Fig. 1G) were incubated with increasing concentrations of STRO-001 for 3 days. STRO-001 exhibited potent anti-leukemia activity against primary AML cells with IC-50s of 17.4nM, 12.8nM, and 4.07nM, respectively (Fig. 1H).
Conclusion: Through transcriptomics profiling and validation of the cell surface expression by flow cytometry, we have identified CD74 as a viable therapeutic target for AML and ALL in children and adults. We further demonstrate that targeting CD74 with STRO-001 effectively eliminates leukemia cells both in vitro and in vivo, providing the preclinical data to compel evaluation of STRO-001 in clinical trials for childhood and adult leukemia.
Hylkema: Moderna: Current equity holder in publicly-traded company; Quest Diagnostics Inc: Current equity holder in publicly-traded company. Pardo: Hematologics, Inc.: Current Employment. Abrahams: Sutro Biopharma: Current Employment. Bedard: Sutro Biopharma: Current Employment. Molina: Sutro Biopharma: Current Employment. Eidenschink Brodersen: Hematologics, Inc.: Current Employment, Other: Equity Ownership. Loken: Hematologics, Inc.: Current Employment, Other: current equity holder in a privately owned company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal